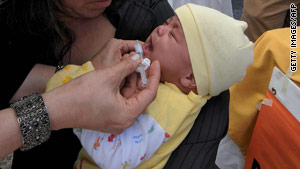
"He couldn't cry - his head was hanging down in the car seat and he couldn't move. I was petrified - it was one of the worst experiences of my life."
Seasonal flu vaccinations across Australia for children under five have been suspended after 23 children in Western Australia were admitted to hospital with convulsions following their injections.
http://www.watoday.com.au/wa-news/flu-vaccination-ban-goes-national-after-fever-convulsions-in-children-20100423-tglp.html
One child, aged 1, remains in a coma in a Perth hospital.
Commonwealth chief health officer Professor Jim Bishop yesterday announced the suspension while authorities urgently review data from around the country.
WA's chief public health officer Tarun Weeramanthri has defended the response time in closing down the state's juvenile flu vaccine program amid revelations that children were presenting with convulsions more than two weeks ago.
* Rogue batch may be to blame
* National warning to GPs
* Perth mum: 'I was petrified'
* WA free vaccine program suspended
More than 60 children around the state may have had adverse reactions to the vaccine, including fevers, vomiting and febrile convulsions - a type of fit brought on by a high fever.
One child remains in a critical condition in hospital after being given the vaccine. Dr Weeramanthri said he had few details on the child's condition but they were "seriously ill".
He said a national process set by the Therapeutic Goods Administration had been observed in responding to the reactions. Under the process the best clinical information was collected from as many doctors as possible and an assessment made on the "totality of that".
"We take all reports very seriously and we believe we've acted in a very timely fashion," Dr Weeramanthri said.
"We've been monitoring the situation, we've been talking to clinicians and we've acted as soon as we can."
He said that since this year's vaccine program started a month ago, 23 children under the age of 10 had presented to Princess Margaret Hospital with convulsions related to vaccinations they had received less than 12 hours before.
Another 40 convulsion cases had been detected in the past month in children at other metropolitan hospitals and in Bunbury. Doctors are now working to determine how many of those children received the flu vaccine.
Aside from the convulsions, affected children were suffering fever and vomiting within 12 hours of their flu shots.
A teleconference today with state, territory and TGA officials confirmed the picture in other states would not be available for "a few days".
Dr Weeramanthri said the TGA was assessing the geographical spread of symptoms across Australia, and directly testing batches of vaccine for any impurity.
Rogue batch may be to blame
Health authorities are also working to determine if the entire Fluvax drug, or just batches, have caused the symptoms, and whether an alternative vaccine should be used.
University of Western Australia school of Paediatrics and Child Health Associate Professor Peter Richmond said that only Fluvax - produced by Australia's biggest biopharmaceutical company CSL - was being used to vaccinate children in WA.
Dr Richmond said researchers were trying to determine whether it was the entire vaccine, or just batches, that had caused the problems which today prompted Australia's chief medical officer to tell doctors to stop giving the vaccine to children.
He said the side effects had been largely limited to children under the age of five and he would not recommend that anybody in other groups - including elderly people - cancel their flu shots.
"This is not a long-term safety issue with vaccines," Dr Richmond told WAtoday.com.au.
He recommended parents of young children who had received only the first of the required two vaccination doses hold off on the second dose for now.
This was despite the fact children who had no side effects from their first dose were unlikely to receive complications from their second.
Dr Richmond said the first dose provided partial protection against the flu anyway.
He said researchers were examining whether an alternative drug to Fluvax could be used for the second dose - generally scheduled for four weeks after the first.
Researchers were also trying to determine if the problem with Fluvax was temporary only - and whether the drug could still be used in coming weeks for the second dose.
He stressed that the vast majority of children receiving Fluvax had suffered no complications.
National warning to GPs
Commonwealth chief medical officer Jim Bishop issued a national warning to GPs not to use the vaccine followed a decision last night by the WA government to suspend the free vaccination program for children under five over concerns it was causing high fevers and convulsions.
"We suggest doctors and health professionals vaccinating children don't use the seasonal flu vaccine for the moment, until we can get the Therapeutic Goods Administration (TGA) to investigate this in more detail," Professor Bishop told ABC TV.
He said the concerns stemmed from a significant rise in the number of children developing a fever after receiving the vaccine.
"We need more information about what’s happened in WA, but also what we can now find out from all the other states from their experience," Professor Bishop said.
"If this has been brought up as a possible side-effect of this drug, then we ought to at least suspend its use until we know more."
In light of the seasonal flu shot suspension, Professor Bishop suggested children get vaccinated against swine flu instead, because that could be a health risk this winter too.
He said there did not appear to be any side effects from the swine flu vaccine Panvax.
"It is safe to have the swine flu vaccine," Professor Bishop said.
"The TGA's assessment of clinical trials and the advice of its expert committees is that Panvax is a safe, effective vaccine for prevention of the H1N1 influenza.
"It is expected that the dominant flu this winter season will be swine flu and the specific Panvax vaccine is available free for all Australians."
'I was petrified'
Perth mother of two Bea Flint said her 11-month-old boy Avery had a seizure after receiving the first dose of the two-dose flu vaccination on Saturday.
Mrs Flint said that after the 9am vaccination she noticed Avery had a minor temperature about 2pm. She treated him with Panadol and by Avery's 7pm bedtime he seemed "OK".
However, at 7.45pm, Avery started whimpering and moaning.
When Mrs Flint got to his cot the baby had vomited and was lying on his side having a seizure.
"In the car driving to the hospital he was just whimpering," she said.
"He couldn't cry - his head was hanging down in the car seat and he couldn't move.
"I was petrified - it was one of the worst experiences of my life."
By the time Avery arrived at St John of God Hospital in Murdoch, he was burning up with a fever of 39.5 degrees.
The doctor who treated Avery told Mrs Flint her baby was the fifth child with similar symptoms admitted to the hospital that day.
WA vaccine program suspended
Health Minister Kim Hames last night advised of the state-wide suspension as a precautionary measure.
He said the suspension came after a significant rise in the number of children who had developed a high temperature after receiving the vaccine.
He said some children had gone into febrile convulsions, a fit caused by a high fever, following the vaccinations.
Dr Hames said it was unclear if the fevers were related to the influenza vaccination but the precautionary measure was the most responsible course of action.
Fevers in most instances are treatable.
"People should give Paracetamol according to the instructions and tepid sponging to keep the temperature down." Dr Hames said.
"On rare occasions children can have a convulsion as the result of the high temperature and sometimes that can be prolonged, which can be a risk to the child."
He said parents should not take children under the age of five to be vaccinated against influenza until further notice.
CLICK HERE TO GO BACK TO MAIN PAGE................or HERE TO GO HOME